Blogs & News
Building trust and improving participation in clinical trials using innovative electronic data capture platforms

Having worked with a number of Electronic Data Capture (EDC) platforms, including Oracle Inform and iMedidata Rave, I have considerable experience of the bugs and intricacies associated with building, cleaning and maintaining electronic Case Report Forms (eCRFs). Not only are many of the market-leading platforms expensive to build within and difficult to edit after go-live, they are also prone to time-wasting bugs. Such flaws are time consuming and expensive to fix – if they can be fixed at all. Thankfully not all my experiences with EDC platforms have been as painstaking.
I joined Aridhia in September and quickly became involved in building eCRFs for a project currently in progress at Stratified Medicine Scotland Innovation Centre. The High Grade Serous Ovarian Cancer study is using Castor EDC, an online, user-friendly portal within which one user can design, build, edit, query and verify eCRFs with ease. The form builder approach allows users to create eCRFs much like you would create a survey in Survey Monkey, however more advanced users can import study structures in xml format if they prefer.  These can be edited after go-live at no additional cost, unlike many other such platforms. Castor’s help desk responded promptly to my queries and provided a wealth of helpful online resources. Castor are very much advocates of collaboration and promoting appropriate sharing of data in a standardised format – much like Aridhia – and they provide a form sharing facility where fellow users can share forms they have built for others to re-use – a very useful tool which in some ways exemplifies how methods of stratified medicine will succeed; through collaboration between different organisations and the sharing of ideas and information.
These can be edited after go-live at no additional cost, unlike many other such platforms. Castor’s help desk responded promptly to my queries and provided a wealth of helpful online resources. Castor are very much advocates of collaboration and promoting appropriate sharing of data in a standardised format – much like Aridhia – and they provide a form sharing facility where fellow users can share forms they have built for others to re-use – a very useful tool which in some ways exemplifies how methods of stratified medicine will succeed; through collaboration between different organisations and the sharing of ideas and information.
Castor incorporates all the features you would expect from a market-leading EDC platform, including:
- Query per field
- Query reports
- Site monitoring functions (source data verification, CRF locking)
- PI sign off
- Report building

Derk Arts, CEO, Castor
speaking at Aridhia’s recent office opening event at the Queen Elizabeth University Hospital in Glasgow
Clinical Trials in Stratified Medicine
From a stratified medicine perspective, patient retention and positive attitudes towards clinical trials are crucial in promoting trial participation, particularly for rare diseases with small gene pools. A bad experience can have a considerable effect on patient retention and institutional trust – from my experiences prior to joining Aridhia, many participants were lost during routine hospital visits due to simple factors such as annoyance at the commute, or the length of the visit, and ultimately withdrew consent from the study. When such factors can have a negative effect on retention rates, the importance of improving and maintaining a positive attitude towards clinical trials and retaining patient trust should not be underestimated. Patients and their data are fundamental to the success of stratified medicine, and if they are to consent to participating in trials they should be well assured that they are contributing to the advancement of medical practice, and not be left feeling unfulfilled or inconvenienced. Furthermore, stratified practices must be generally accepted to address the requirement for leveraging clinical trials with genomic data to improve drug development and reduce adverse drug reactions. I was pleased to find that Castor provides features which address such problems. For example, patient questionnaires can be built within Castor and distributed via email at scheduled intervals (e.g. Day 1, Day 2, Day 29, End of Treatment) in order to reduce the requirement for patients to attend a treatment centre simply to complete an EQ-5D questionnaire.
Below is an example of an EQ-5D questionnaire which I built within Castor:
Patient email

Online Survey View
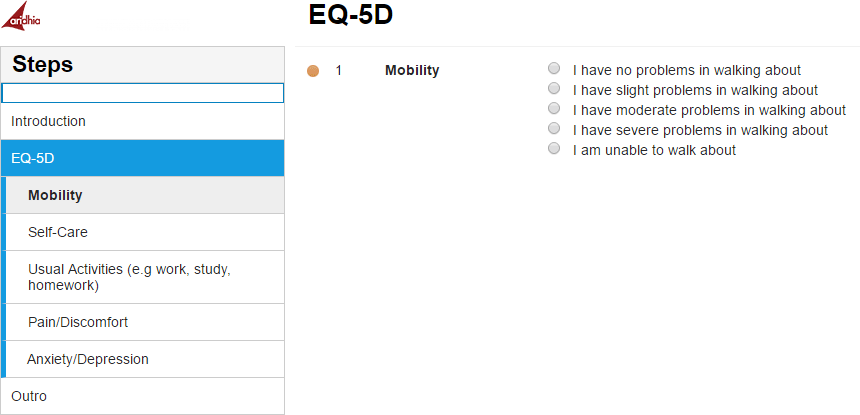
Given that features like this are available at a reasonable cost alongside full user configurability and with little need for help desk involvement, some may find Castor a more attractive option than rival products. Such features have obvious benefits in an environment in which requirements can change rapidly, enabling both small research groups and larger institutions to easily manage their own updates quickly, safely, efficiently and cost-effectively.
Scepticism and Acceptability towards Stratified Medicine
Here in Scotland, while the Data Protection Act does not prevent the linkage of medical data without patient consent, any linkage work must be within the public interest and have associated benefits. Providing patients with evidence of success – by ‘bringing them to the data’ with mini-apps or allowing direct participation in data capture for example – and fulfilling experiences within clinical trials can only improve attitudes and remove scepticism towards stratified medicine. Research suggests that patients are more likely to participate in trials if they are promised feedback on the results and know how their data is being used. We have seen this first-hand at a few of our data challenge events, where patient participants have commented on how pleased they are to see their data being shared and used for clinical research. Mechanisms of informed consent should also be brought in line with the unprecedented amount of data sharing that now occurs due to advancements in information technologies. Biomedical research relies heavily upon bio-repositories and data banks. The amount of information that is now shared digitally is remarkable, however mechanisms of informed consent remain laborious, paper-based, and have limitations when consent for future use of a sample is required. Dynamic consent is a concept that should be embraced to place participants at the centre of decision making in real-time. This would allow a participant to determine the information they share via a digital interface. Ultimately, this would engage patients with the research and make the prospective and retrospective use of biobank samples for example, much easier for research purposes.
If improvements in how patient participation is managed can be achieved, widespread data capture and linkage could become a feature of public interest and improve trial participation. The innovative features offered by platforms such as Castor can aid this process by removing unnecessary patient visits from the clinical trial schedule, bringing patients to the data, and ultimately improving the acceptability of data sharing. Moreover, if more genetic data can be re-used and shared, we may uncover some of the currently hidden genetic heritability of disease. Transforming patient scepticism into trust will go a long way towards accelerating research and paving the way for data sharing, collaboration and trial participation to become the norm.
January 10, 2017
kieran
Kieran joined Aridhia September 2016 with an MSc in Stratified Medicine and Pharmacological Innovation. As an advocate of genetic stratification in medicine and clinical trials, Kieran is excited to work at the forefront of stratified medicine here at Aridhia and is proud of the collaboration and research that Scotland offers to the medical community. Outside of work he plays American football.
