Blogs & News
Enablement of hospital-based precision dosing with the DRE
N.B. You can find the full, published text for this article at the bottom of this blog.
Enablement of hospital-based precision dosing with the DRE
Dose adjustment and/or dose individualization beyond the guidance recommended by the drug manufacturer is part of a modern precision medicine strategy. Using models to guide dosing in such cases leveraging available data an augmenting model-based prediction by including available patient-specific data has been an approach used in a few, mostly research settings for some time. Precision dosing is an approach to utilize various patient-specific data sources to individualize pharmacotherapy of critical medicines used in the care of disease and other conditions for which drug therapy is recommended. Often the “data” in question refers to therapeutic drug monitoring of drug concentrations in blood or plasma. More recently, biomarkers and clinical outcomes have been utilized to further guide dose individualization for critical pharmacotherapy. One of the challenges that many institutions seeking to develop such solutions face is the fact that the input data of interest may not all reside in the same data location and not all are captured in sufficient detail in the electronic medical records. Moreover, different data systems, locations and governance may make the assembly of accurate, real-time data assembly and subsequent analysis particularly challenging.
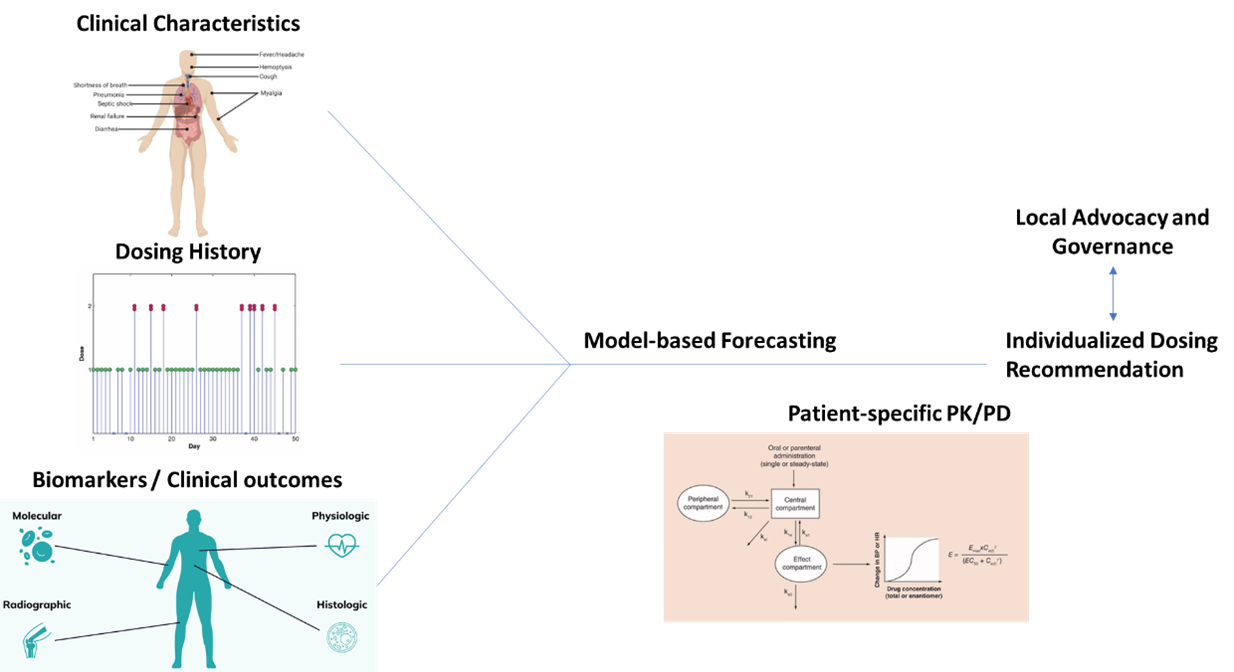
Idealized schematic for the incorporation of essential data towards developing an MIPD strategy and individualized dosing recommendations.
Aridhia’s Digital Research Environment (DRE) provides a secure collaborative research environment for digital analysis of data. Essential companions to the DRE are dynamically updated and searchable metadata catalogs, in situ analysis tools with code versioning, as well as data provenance, and audit trails. These features facilitate the collaboration but also make it compatible with regulatory requirements. One of the more longstanding implementations of the DRE has been at the Great Ormond Street Hospital (GOSH) in London, UK. Specifically the DRIVE (Data Research, Innovation and Virtual Environments) initiative from GOSH provides a state-of-the-art unit dedicated to innovation through data and digital technologies, with partnerships across the NHS, industry and academia. While a central component of DRIVE is collaboration, an additional effort with the DRE implementation at the hospital (led by several UCL scientists) is the use of the DRE coupled with pharmacometric models to explore precision dosing solutions for the patients that walk through the door.
A tremendous opportunity exists with the enablement of precision dosing strategies within the capabilities of a DRE. The two distinct advantages I see are flexibility and collaboration driven. It is a benefit to patients in general to have dosing guidance informed by diverse patient populations (certainly beyond the boundaries of a single institution). This goes beyond the drug model as the standards of care are different around the world as well as the global marketplace for available treatments. Why not benefit from this collective knowledge? Also, for LMICs and geographic regions with limited infrastructure, sharing and collaborative environments is a means to normalize the knowledgebase and experience.
You can find the full text for the publication below.
(If you are viewing on your phone, you can download the pdf here instead.)
References:
- • Wang Y and Goswami S. Understanding FDA’s Perspective on Precision Dosing. Applied Clinical Trials 2022.
- • Barrett JS. Pediatric models in motion: Requirements for model-based decision support at the bedside. British J Clin Pharmacol 79(1):85-96, 2015.
- • Derendorf, H., Peloquin, C. Roger W. Jelliffe, M.D. (1929–2020). Clin Pharmacokinet 59, 1063 (2020).
- • Dombrowsky E, Jayaraman B, Narayan M, Barrett JS. Evaluating Performance of a Decision Support System to Improve Methotrexate Pharmacotherapy in Children with Cancer. J. Ther. Drug Monitoring 33(1): 99-107, 2011.
- • Jelliffe RW, Iglesias T, Hurst A, Foo K, and Rodriguez J: Individualizing drug dosage regimens: comparison of two types or pharmacokinetic models of gentamicin, three methods of fitting serum level data, and several monitoring strategies; Clinical Pharmacokinetics, 21(6):461-478,1991.
- • Jelliffe RW, Schumitzky A, Van Guilder M: A simulation study of factors affecting aminoglycoside therapeutic precision. Drug Invest. 4 (1):20-29,1992.
- • Larkindale J, Betourne A, Borens A, Boulanger V, Theurer Crider V, Gavin P, Burton J, Liwski R, Romero K, Walls R, Barrett JS. Innovations in Therapy Development for Rare Diseases Through the Rare Disease Cures Accelerator-Data and Analytics Platform. Ther Innov Regul Sci. 2022 Jun 6. doi: 10.1007/s43441-022-00408-x. Epub ahead of print. PMID: 35668316.
- • Barrett JS., Betourne A, Walls R, Borens A, Roddy W, Lasater K, Russell S. The future of Rare Disease Drug development: the Rare Disease Cures Accelerator Data Analytics Platform. J Pharmacokinet Pharmacodyn. 2023 May 2. doi: 10.1007/s10928-023-09859-7. PMID: 37131052.
- • Frymoyer A, Schwenk HT, Zorn Y, Bio L, Moss JD, Chasmawala B, Faulkenberry J, Goswami S, Keizer RJ, Ghaskari S. Model-Informed Precision Dosing of Vancomycin in Hospitalized Children: Implementation and Adoption at an Academic Children’s Hospital. Front Pharmacol. 2020 Apr 29;11:551. doi: 10.3389/fphar.2020.00551. PMID: 32411000; PMCID: PMC7201037.
- • Barrett JS, Barrett RF, Vinks AA. Precision Dosing in Children. J Clin Pharmacol. 2021, 61(S1): S36–S51.
- • Kantasiripitak W, Van Daele R, Gijsen M, Ferrante M, Spriet I, Dreesen E. Software Tools for Model-Informed Precision Dosing: How Well Do They Satisfy the Needs? Front Pharmacol. 2020 May 7;11:620. doi: 10.3389/fphar.2020.00620. PMID: 32457619; PMCID: PMC7224248.
- • Green, S., Prainsack, B. & Sabatello, M. Precision medicine and the problem of structural injustice. Med Health Care and Philos 26, 433–450 (2023).
January 12, 2024
jeff
Dr. Jeff Barrett is the Chief Science Officer at Aridhia promoting healthcare and life science partners to collaborate, access and share secure data to deliver better patient outcomes. Before Aridhia, he was Senior Vice-President at the Critical Path Institute serving as the Executive Director of the Rare Disease Cures Accelerator, Data Analytics Platform. Jeff was previously Head of Quantitative Sciences at the Bill & Melinda Gates Medical Research Institute. Prior to MRI, he was Vice President, of Translational Informatics at Sanofi Pharmaceuticals. Jeff spent 10+ years at the University of Pennsylvania where he was Professor, Paediatrics and Director, Laboratory for Applied PK/PD at the Children’s Hospital of Philadelphia.
