Blogs & News
Facilitating Project Team Dynamics with a DRE
Within pharmaceutical development, the project team is the engine that drives the progression of new drugs, devices or vaccines. While there may be additional decision-making bodies that receive recommendations from the project teams and focus on more of the strategic or business aspects of development, the project teams are typically responsible for the development plans, the achievement of critical project milestones and the project budget and timelines. Project teams are supported by multidisciplinary team members representing various functional groups with R&D and Commercial Operations. Figure 1. below provides some of the typical functional groups representing early stage project teams.
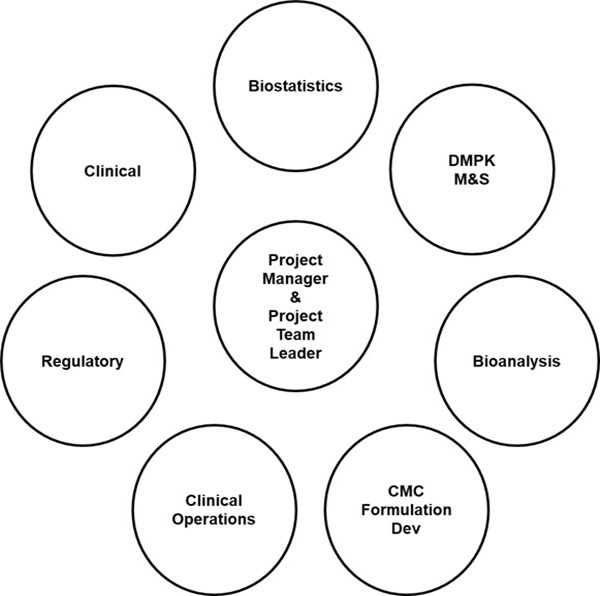
Fig 1. Influential stakeholders that drive and support project team creation, dissolution and project interactions
Within the workings of any project team there are numerous activities that are advanced by the various project team members under the coordination of the project manager supporting the team and the direction of the project team leader(s) [Zeller 2002]. These activities are tracked in the project team’s meeting minutes and captured in coordination with project timelines on Gantt charts (a type of bar chart that illustrates a project schedule). The output from these activities includes data, documents, plans, code and decision outcomes captured chronologically and often simply stored in an unaudited manner, leaving the project team’s memory to cobble together the decision-making history from these documents, reporting the synthesis to senior decision makers periodically [Jekunen 2016]. Quite often the source documents are simply stored on shared drives or perhaps in a SharePoint site. Occasionally, external SMEs are invited to project team meetings as they may contribute guidance on decision-making or perform certain project team tasks (e.g., sample size assessments, simulations, competitive intelligence). In this case the project team also has to manage the security around data and/or document sharing as well as team engagement.
Streamlining Decisions
The figure below illustrates a generalized workflow of project team actions driving to a decision point with the typical interplay between governing bodies and R&D hierarchy for early, late stage and commercial interests. The influence of these bodies changes with the stage of development and the various milestones under evaluation at any given time. The emphasis of the team is to drive the process to make informed recommendations for all Go / No Go decision milestones. Subject matter experts (SMEs) both with and external to the company are often also brought in to help define the target product profile (TPP) and identify patient populations of interest. As each project team is required to provide periodic updates to other decision-making bodies, addressing these items with some frequency ensures that the update is current and reflects all key inputs for transparency in decision-making.
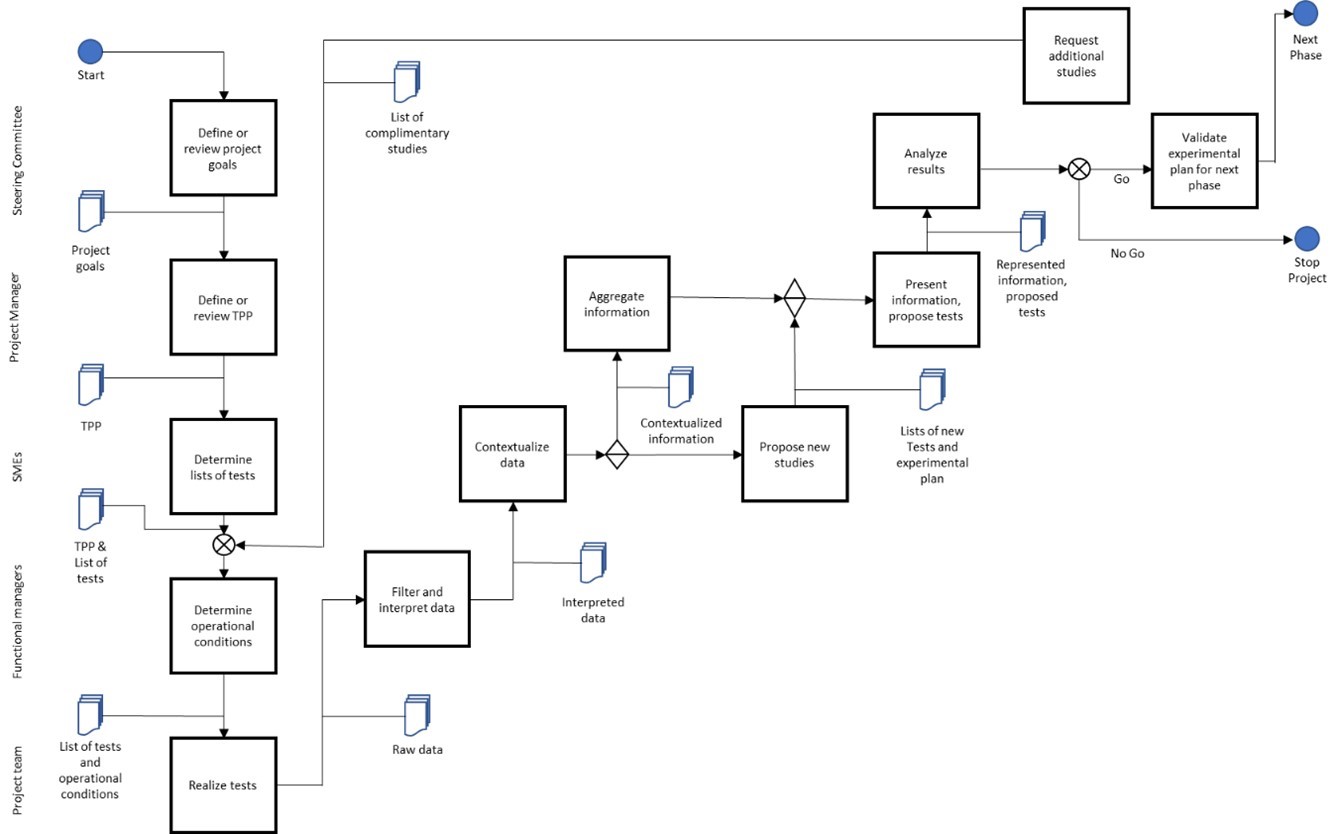
Fig 2. Example workflow of project team activities in coordination with stakeholder input and review of recommendations prior to decisions on milestone achievement.
Aridhia’s Digital Research Environment (DRE) may be a reasonable solution for Project Teams improving efficiency and facilitating the construction of regulatory submission data and documents in a more transparent and auditable manner. There’s been immense interest in leveraging digital cloud solutions in this space, and we’ve recently been working with several biopharma groups to use the DRE as a secure collaborative research environment for digital analysis of data.
Essential components of the DRE are dynamically updated and searchable metadata catalogues, in situ analysis tools with code versioning, as well as data provenance, and audit trails. These features facilitate not only collaboration but project team interaction and also make it compatible with regulatory requirements. The DRE is also agnostic to various other legacy solutions employed by PhRMA sponsors such as LIMS systems, document management systems or compute environments offering ease of integration and a mechanism to leverage utility of these legacy systems, not requiring training on yet a new toolset.
Management of SME engagement is easily facilitated within such a trusted research environment as well. SMEs can be granted secure access for a finite period of time with certain access and privileges defined by their contract and expected role. Access can expire at a prescribed time or be rescinded at a moment’s notice, providing the sponsor with the ultimate control. Conditions of workspace access and usage can likewise be specified in contractual agreements so that expectation are clearly defined at the beginning of such relationships.
Project Evaluation
At some point in the development process there may be a need to dissolve the project team and move the team members to other projects [Barrett 2022]. Reasons for project team termination can include any of the following:
- Disappointments in a product (unwanted side effects or marginal efficacy)
- Change in product environment (internal and external competition)
- Change in financing
Likewise, there are preplanned evaluations for consideration of project team termination based on key milestones timing that coincides with financial investment increases. Evaluations are usually made by one of the aforementioned governance bodies. Typically, these evaluation periods occur after Phase 0 (< 15 subjects given a very small dose of a compound to make sure it isn’t harmful to humans before higher doses are administered), after Phase I–II and before Phase III. Of course, at any time, information regarding a development compound becomes known that suggests a low probability of development success, a candidate may be abandoned, and the project team disbanded. Luckily, this simply means a new opportunity for the company and a new project team for the team members. The reality for the output generated by project teams in this situation (project team dissolution) is that it simply may become archived or worse yet simply forgotten about, taking up file space with those knowledgeable on the project assigned to new tasks.
Here again, the DRE can be invaluable in a variety of ways. By capturing the key progression milestones, personnel engaged and decision criteria, the DRE workspace’s audit trail provides a more robust record of project team event chronology without searching through agendas and meeting minutes or relying on members’ memory. The governance supporting DRE workspace access, data ingestion, data sharing and analyses is easily managed via designated roles that can exist on the project team already maintaining project familiarity (see Figure 3 below). Moreover, in the event the project continues with subsequent studies and phases of development, workspace content can be cloned, keeping key data and documents (e.g., protocols, Investigator Brochures, SAP, latest population PK/PD model).
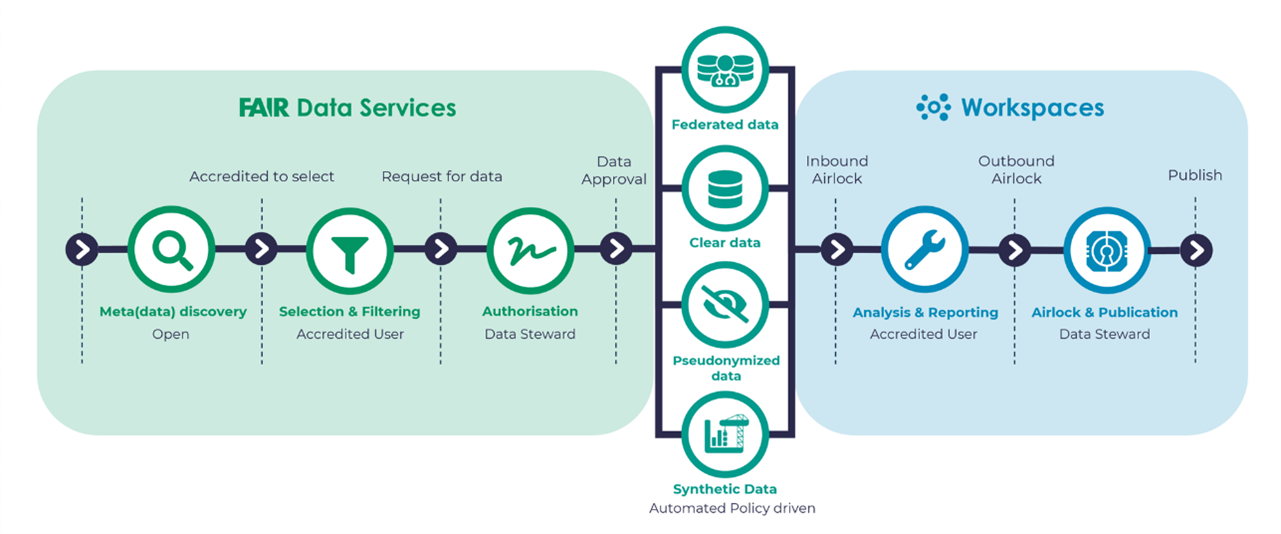
Fig 3. Interplay between FAIR Data Services and Workspaces integration and governance that would support project team interactions during drug development
A key component of the workspace interaction is also the interaction with FAIR Data Services that are also an important component of the Aridhia DRE. Recognizing that early and late phase drug development deals with proprietary, non-proprietary and patient-level data, the FAIR (findable, accessible, interoperable and reusable) aspect of the environment is essential to ensure that metadata tags are in place and that data eligible for regulatory submission is appropriately curated with relevant standards (e.g. CDISC) applied. Applying the FAIR principles also opens new opportunities by making retrospective study and analysis for past trials and regulatory submissions available in a digitized and searchable format for future researchers, recognizing the value of legacy data and information.
References:
- • Jekunen A. Decision-making in product portfolios of pharmaceutical research and development – managing streams of innovation in highly regulated markets. Drug Des Devel Ther. 2014; 8: 2009–2016.
- • Zeller C. Project Teams as Means of Restructuring Research and Development in the Pharmaceutical Industry. Regional Studies, Vol. 36.3, pp. 275–289, 2002.
- • Barrett JS, editor, Fundamentals of Drug Development, Wiley; 1st edition (September 7, 2022), ISBN-10: 1119691699, ISBN-13: 978-1119691693 (512 pages).
January 25, 2024
jeff
Dr. Jeff Barrett is the Chief Science Officer at Aridhia promoting healthcare and life science partners to collaborate, access and share secure data to deliver better patient outcomes. Before Aridhia, he was Senior Vice-President at the Critical Path Institute serving as the Executive Director of the Rare Disease Cures Accelerator, Data Analytics Platform. Jeff was previously Head of Quantitative Sciences at the Bill & Melinda Gates Medical Research Institute. Prior to MRI, he was Vice President, of Translational Informatics at Sanofi Pharmaceuticals. Jeff spent 10+ years at the University of Pennsylvania where he was Professor, Paediatrics and Director, Laboratory for Applied PK/PD at the Children’s Hospital of Philadelphia.
