Blogs & News
How the DRE Accelerates Regulatory Impact of Consortium-Based Projects
A new paper published last week in Nature Reviews Drug Discovery highlights strategies to enhance the regulatory impact of consortium-based projects. Published by Critical Path Institute® (C-Path) and Innovative Health Initiative (IHI), the paper outlines key strategies that consortium-based projects can use to significantly enhance their regulatory impact, ultimately accelerating the development of new therapies.
C-Path has established numerous international consortia, programs and initiatives that currently include more than 1,600 scientists and representatives from government and regulatory agencies, academia, patient organisations, disease foundations and pharmaceutical and biotech companies.
Drawing on this experience, key lessons and recommendations include:
• Embedding regulatory thinking from the start, not as an afterthought.
• Building and governing data infrastructure with regulatory reuse and sustainability in mind.
• Operating under neutral facilitation to align various interests.
• Engaging regulators early to ensure the resulting tools and data qualify for actual regulatory use.
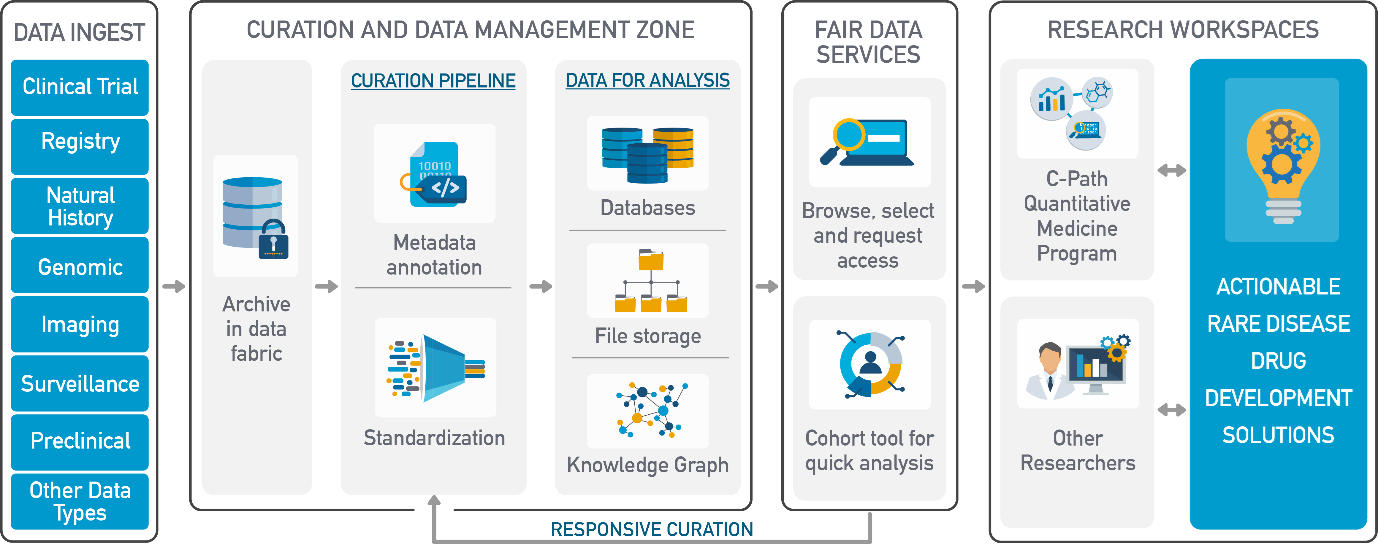
C-Path’s Data and Analytics Platform
As a globally recognised pioneer in accelerating drug development, C-Path’s mission is to lead collaborations that advance better treatments for people worldwide. Facilitating public-private partnerships in conjunction with a robust Data Collaboration Center and Quantitative Medicine program, C-Path works to standardise data and create tools for researchers and drug developers to utilise on their secure cloud-based platform, the Rare Disease Cures Accelerator-Data and Analytics Platform (RDCA-DAP®).
Powered by the Aridhia DRE, RDCA-DAP is designed to speed up the development of treatments for rare and orphan diseases using…
• FAIR Data Services: Integrated catalogue of rare disease data and drug development tools (DDTs) from multiple sources.
• Workspaces: Secure sandboxes for collaborative data interrogation, advanced analytics, and model development in real-time.
How the Aridhia DRE Supports Regulatory Goals
1. Scalable Data Infrastructure
Centralised data and tooling catalogue: FAIR Data Services provides a comprehensive repository of patient-level data (trials, registries, and real-world sources) and tools that are easy to browse, preview, and request access.
Standardisation-ready: Aligning with ‘FAIR’ principles (Findable, Accessible, Interoperable, Reusable) and CDISC standards, FAIR enables regulatory-grade data sharing.
Scalable & sustainable: FAIR allows data owners to define the terms of use for their data, manage and automate data access rules, and track data use with detailed audit and platform metrics over time.
2. Secure Multi-Stakeholder Collaboration
Role-based access control: Allows different levels of access for pharma, regulators, academics, and patient advocates, enabling trust while maintaining confidentiality.
Governance workflows: Track data/tooling versions, usage, and approvals within a neutral, rule-based framework designed for easily managing the complexities of cross-sector governance.
Audit-ready environment: Important for accountability and compliance, data lineage, usage logs, and permissions are transparent and make it easy for data owners and administrators to track.
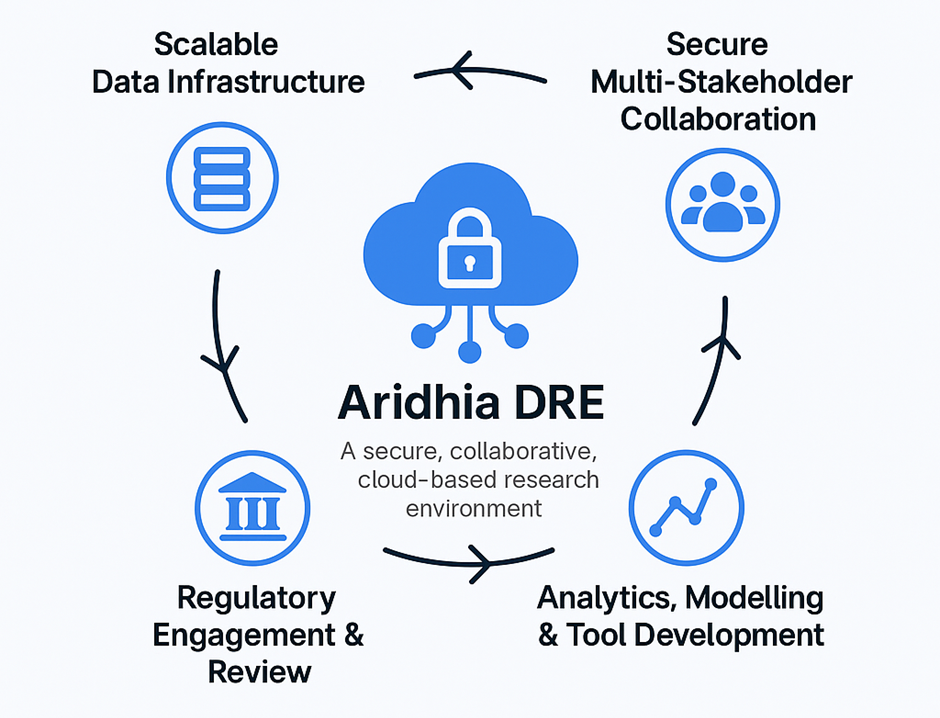
3. Analytics, Modelling & Tool Development
Collaborative environments: Aridhia Workspaces allow real-time collaboration across geographically distributed teams and come stocked with pre-installed tools and Git versioning for reproducibility.
Analytical workspaces: Researchers can collaboratively develop, test, and validate biomarkers, endpoints, or disease progression models in private sandbox spaces – all by using C-Path data/tools or bringing their own.
Rapid iteration: Intermediate results can be safely ‘Airlocked’ to share with other workspaces to get real-time feedback from regulators without compromising data integrity or IP.
4. Regulatory Engagement & Review
Submission-ready outputs: Using the Aridhia DRE, exportable, standards-compliant evidence packages can be generated to support regulatory submissions – these can be programmed to follow organisational processes or use open-source tools like pharmaverse.
Regulatory access: Review workspaces can be spun up within minutes for regulatory authorities to view or interact with data/tools in the DRE, streamlining feedback loops and providing feedback opportunities at different stages.
Provenance tracking: Supports validation and regulatory scrutiny by documenting how every dataset, model, or output was created through detailed audit and built-in Git versioning.
Putting Strategy into Action
Following this approach, the Aridhia DRE transforms C-Path and IHI’s regulatory playbook into a practical, secure, and sustainable digital workflow.
It serves as the operational core, enabling consortiums to plan, build, analyse, and qualify drug development tools in line with regulatory expectations, and effectively turns strategy into scalable impact.
By using RDCA-DAP, C-Path gives their consortia the tools to move from vision to regulatory reality and put their mission into action: accelerate the development of new therapies and improve patient outcomes.
June 25, 2025
Kara Lasater
Kara joined Aridhia in 2020 and leads the Customer team as Head of Customer Success where she works closely with clients to deliver valuable solutions on the DRE. Kara leads with a commitment to understanding and addressing customer needs and fostering a customer-centric culture at Aridhia. In her free time, she enjoys hiking and taking road trips with her family and labradoodle, Cosmo.