Blogs & News
How Australia’s Liver Cancer Collaborative is Transforming Diagnosis and Precision Treatment with the Aridhia DRE
Aridhia have recently partnered with the Liver Cancer Collaborative (LCC) in Australia to transform research and analysis of multi-modal liver cancer data and improve diagnosis and outcomes for patients. Through generous funding from the Cancer Research Trust and other partners, the LCC, along with Perkins Cancer Biobank, are building one of the world’s most extensive tissue repositories for liver cancer. These samples are being used across major research programs, including full exome (DNA) and transcriptome (RNA) sequencing on the tumour samples to identify new biomarkers for liver cancer and building laboratory models of liver cancer including patient-derived organoids (a 3D replica of the tumour in a dish) and precision medicine.
To complement this, LCC are developing a secure Digital Research Environment that will provide a platform for sharing data generated through these research programs. In this state-of-the-art discovery and patient-focused research pipeline, detailed clinical data will be combined with research data and access will be made available to bio-samples with the aim to developing precision medicine for liver cancer. As usage of the platform increases, clinical collaborators will contribute to a clinically responsive database to assist with clinical decision making.
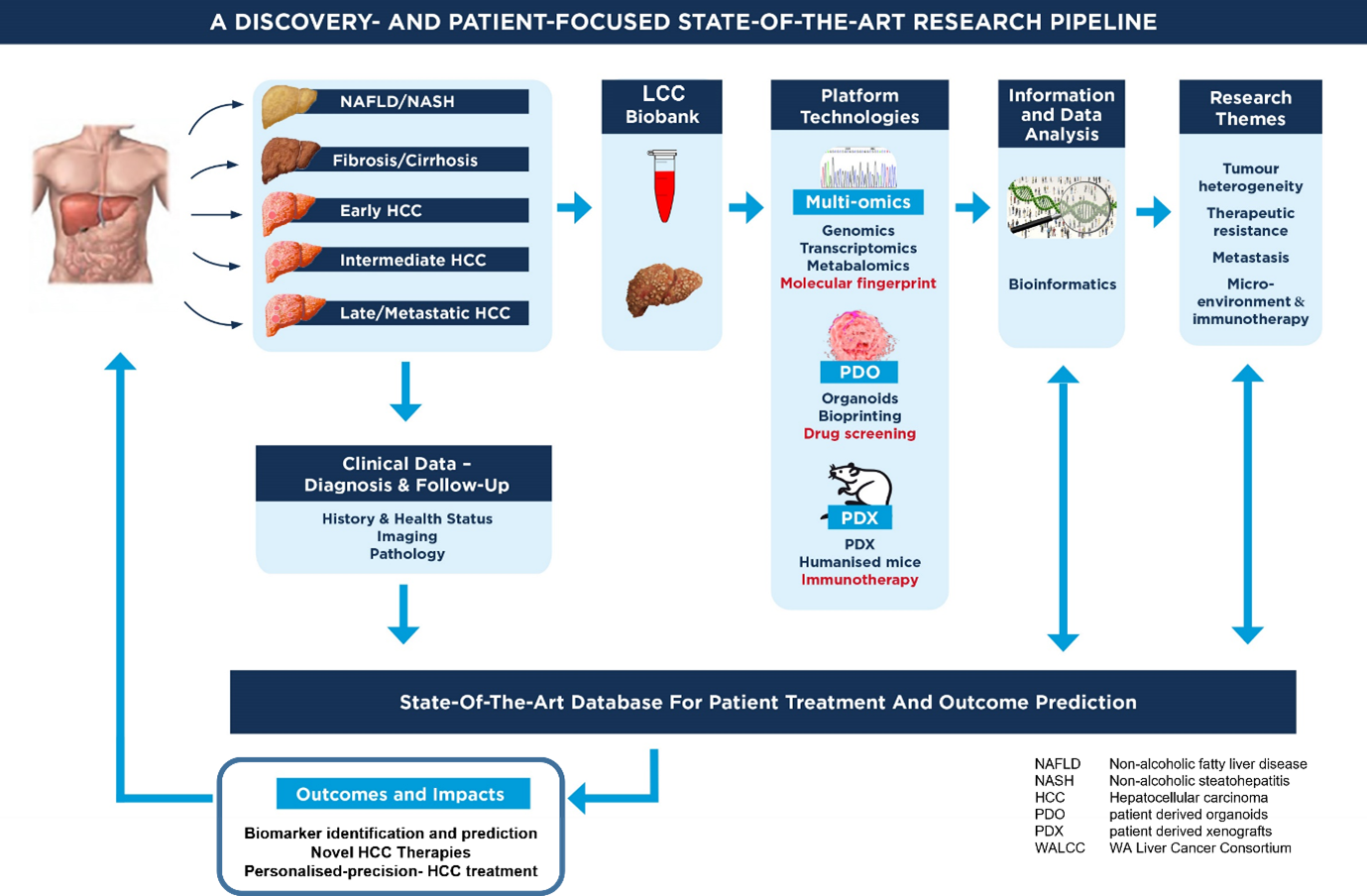
A Platform Underpinned by the DRE
This platform allows researchers, for the first time, to combine patient-linked clinical, genomic sequencing data and radiology imaging data in a single secure collaborative workspace, with bespoke tooling tailored for data exploration and analysis.
The Aridhia DRE provides a full end-to-end, enterprise-scale digital research environment. FAIR Data Services, provides a ‘shop window’ of data: presenting detailed catalogue and dictionary information for researchers, and provided an extensive cohort builder tool, which allows researchers to understand what data is available for their area of study, and then request access to a multi-modal subset of data relevant to their project.
A fully orchestrated data approval workflow allows for a consistent and traceable data access request process. Upon approval, researchers have access to the appropriate cohort of data within their Aridhia DRE collaborative workspace. This will include de-identified clinical data for the patients requested, access to appropriate genomics sequencing data and, by integrating with OpenSpecimen and combining clinical data with bio-sample references, researchers can request targeted physical samples relevant to their identified cohort of data.
The LCC platform workspace is pre-loaded with tooling tailored to the users, with access to RStudio, Jupyter Notebook, bespoke LCC-provided R Shiny applications for data exploration and clinician-focused analysis, out of the box analytics and complemented by the ability to spin up virtual machines of various configurations: providing researchers with the facility to bring their own tools. The workspace provides inbound and outbound airlocks, ensuring data cannot enter or leave the workspace without approval, and in-built Gitea for software versioning control.
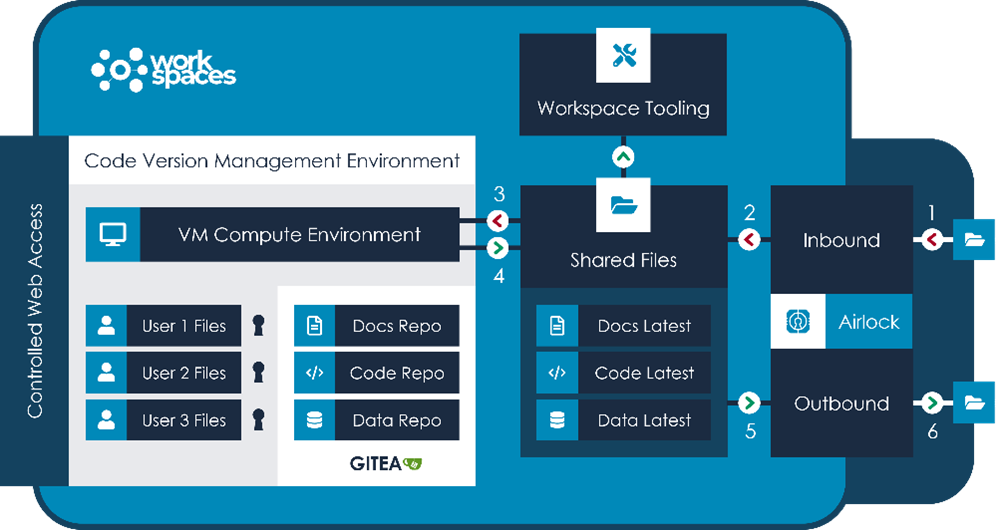
A schematic of a workspace, showing data flow from inbound airlock, to that data used within a secure compute environment, to outbound airlock
For further collaboration, LCC has provided a means within their DRE platform for researchers to broadcast information on their research and data used. Users of the platform can browse projects and research and are encouraged to collaborate and work with others.
Some examples of the types of analysis that LCC users will perform with the Aridhia DRE Workspaces are:
- Identify biomarkers that can predict the response to different therapies (such as immunotherapy or targeted therapy) and personalise treatment plans for each patient.
- Compare the genomic profiles of primary and metastatic tumours, and understand how liver cancer evolves and develops resistance to drugs.
- Explore the associations between clinical variables (age, gender, ethnicity, lifestyle factors, liver cancer risk, etc.) incidence and survival.
- Visualise and quantify the tumour characteristics, such as size, shape, density, vascularisation and heterogeneity, using advanced imaging techniques and machine learning algorithms.
- Link the molecular and imaging data with the physical samples, such as blood, tissue and urine, and track the provenance and quality of the samples throughout the research process.
Using the Aridhia DRE platform, providing access to curated, patient multi-modal data and bio samples can lead to outcomes that will benefit the understanding of liver cancer, such as the discovery of new molecular targets and pathways that can be exploited for drug development or repurposing. It will identify new risk factors and prevention strategies that can reduce the burden of liver cancer in the population.
Crucially, this research will improve the accuracy and efficiency of liver cancer diagnosis and staging and reduce the need for invasive procedures while allowing for enhanced monitoring and evaluation of treatment response and progression, allowing clinicians to adjust the treatment accordingly.
A Precision Approach
Finally, increased collaboration and communication among researchers and clinicians across different disciplines and institutions will foster a multidisciplinary approach to liver cancer research and ultimately lead to better patient outcomes and successful treatment.
Louise Winteringham, Program Manager at Liver Cancer Collaborative and Head of the Translational Cancer Research Program at Perkins Cancer Biobank, summarises: “For research data to contribute meaningfully to clinical decision making it must be informed by the patient’s journey. Aridhia’s platform provides a highly secure and uniquely collaborative space where we can analyse complex research data together with a patient’s clinical information. Combining these data is critical to developing a precision approach to treating liver cancer.”
January 17, 2024
Scott Russell
Scott joined Aridhia in March 2022 with over 25 years’ experience in software development within small start-ups and large global enterprises. Prior to Aridhia, Scott was Head of Product at Sumerian, a data analytics organisation acquired by ITRS in 2018. As CPO, he is responsible for product capabilities and roadmap, ensuring alignment with customer needs and expectations.
